Novel Coronavirus (COVID-19) Infection : Sign & Symptoms, Transmission, Risk factors, Diagnosis, Complication, Treatment and Prevention….
2019 Novel Coronavirus (2019-nCoV) is a virus (more specifically, a coronavirus) identified as the cause of an outbreak of respiratory illness first detected in Wuhan, China. Early on, many of the patients in the outbreak in Wuhan, China reportedly had some link to a large seafood and animal market, suggesting animal-to-person spread. However, a growing number of patients reportedly have not had exposure to animal markets, indicating person-to-person spread is occurring. At this time, it’s unclear how easily or sustainably this virus is spreading between people. The latest situation summary updates are available on CDC’s web page 2019 Novel Corona virus, Wuhan, China.
Coronaviruses are a large family of viruses that are common in many different species of animals, including camels, cattle, cats, and bats. Rarely, animal coronaviruses can infect people and then spread between people such as with MERS, SARS, and now with 2019-nCoV. CDC is closely monitoring an outbreak of respiratory illness caused by a novel (new) coronavirus (named “2019-nCoV”) that was first detected in Wuhan City, Hubei Province, China and which continues to expand. Chinese health officials have reported tens of thousands of infections with 2019-nCoV in China, with the virus reportedly spreading from person-to-person in parts of that country. Infections with 2019-nCoV, most of them associated with travel from Wuhan, also are being reported in a growing number of international locations, including the United States. Person-to-person spread of this virus outside China has been detected.
The United States reported the first confirmed instance of person-to-person spread with this virus on January 30, 2020. On January 30, 2020, the International Health Regulations Emergency Committee of the World Health Organization declared the outbreak a “public health emergency of international concern external icon” (PHEIC). On January 31, 2020, Health and Human Services Secretary Alex M. Azar II declared a public health emergency (PHE) for the United States to aid the nation’s healthcare community in responding to 2019-nCoV. Also on January 31, the President of the United States signed a presidential “Proclamation on Suspension of Entry as Immigrants and Non-immigrants of Persons who Pose a Risk of transmitting 2019 Novel Coronavirus external icon“. These measures were announced at a press briefing by members of the President’s Coronavirus Task Force external icon.
Chinese health authorities were the first to post the full genome of the 2019-nCoV in Gen Bank external icon, the NIH genetic sequence database, and in the Global Initiative on Sharing All Influenza Data (GISAID external icon) portal, an action which has facilitated detection of this virus. CDC is posting the full genome of the 2019-nCoV viruses detected in U.S. patients to GenBank as sequencing is completed. 2019-nCoV is a beta-coronavirus, like MERS and SARs, both of which have their origins in bats.
The sequences from U.S. patients are similar to the one that China initially posted, suggesting a likely single, recent emergence of this virus from an animal reservoir. Early on, many of the patients in the outbreak of respiratory illness caused by 2019-nCoV in Wuhan, China had some link to a large seafood and live animal market, suggesting animal-to-person spread. Later, a growing number of patients reportedly did not have exposure to animal markets, indicating person-to-person spread. Chinese officials report that sustained person-to-person spread in the community is occurring in China. Person-to-person spread has been reported outside China, including in the United States and other countries. Learn what is known about the spread of newly emerged coronaviruses.
Worldwide scenario of Coronavirus Infection
Globally, on the 30th January 2020 the World Health Organization (WHO) of the United Nations declared the pandemic a public health emergency of international concern. In 2020 as upto on 28th March, WHO was discovered globally, Corona virus infecting 663748 people and killing 30880 people. This defines the outbreak an “extraordinary event which is determined to constitute a public health risk to other States through the international spread of disease and to potentially require a coordinated international response”. Several countries and airlines immediately suspended travel from affected areas, closed some borders with China, and initiated comprehensive preventative screening at airports. “on the 30th January 2020 the World Health Organization (WHO) of the United Nations declared the pandemic a public health emergency of international concern”. Early on, the Chinese authorities revealed that the outbreak was caused by a novel beta-coronavirus, named 2019-nCoV. This was soon found to be significantly genetically related to SARS-CoV and other bat coronaviruses.
Globally, as on 17 April 2020, a total of 2231438 have been reported worldwide; These include 564718 who have been cured/discharged and 15837 deaths. This condition have had gradually goes to more severe, and as on 6 May 2021, there have been 154,815,600 confirmed cases of COVID-19, including 3,236,104 deaths, reported to World Health Organization.
The initial cases were associated with a seafood market in Wuhan where live animals were sold. This suggested a possible animal reservoir for this virus and suggested zoonotic (animal to human) transmission. Later on, environmental samples obtained from this market were found to be positive for 2019-nCoV, strengthening the hypothesis that this is a zoonotic virus. However, whilst the virus was found to have originally started among infected animals, most of the subsequently reported cases were shown to be caused by human-to-human transmission.
Beta-coronaviruses have caused major epidemics in the last 2 decades. In 2003, SARS-CoV was discovered in China before being spread globally, infecting 8,098 people and killing 774. This was later found to be zoonotic in origin and thought to have started amongst a bat reservoir before later infecting wild civet cats and raccoon dogs that were being sold at live wild animal markets destined for human consumption. In 2012, MERS-CoV was discovered in Saudi Arabia before also spreading globally resulting in 2506 confirmed cases and killing 862 people worldwide. Again, this started as a zoonotic virus, shown to move from camels to humans and again thought to have initially emerged from bats. Imported cases of 2019-nCoV infection in travellers have been detected in the U.S. Person-to-person spread of 2019-nCoV also has been seen among close contacts of returned travellers from Wuhan, but at this time, this virus is NOT currently spreading in the community in the United States.
Indian scenario of Coronavirus Infection
India reported the first confirmed case of the coronavirus infection on 30 January 2020 in the state of Kerala. The affected had a travel history from Wuhan, China. Two more cases were also confirmed in Kerala and are reported to be clinically stable. The Indian government arranged for the evacuation of 366 Indian citizens from Wuhan in a special Air India flight on 31 January 2020. The passengers are placed under quarantine for a period of 14 days. A second batch of 330 passengers, including seven Maldivian citizens evacuated by the government, arrived from Wuhan on 01 February 2020. All the passengers are currently being monitored. Thermal screening has been installed at all major international airports in India, including those in Delhi, Mumbai, Kolkata, Chennai, Bengaluru, Hyderabad, and Cochin. As on 06 February 2020, 138,750 passengers from 1,265 flights have been screened at various Indian airports.
23 states/UTs including New Delhi have issued orders allowing only essential services to operate in 75 districts with confirmed COVID-19 cases until 31 March 2020. The focus is on closure of all activities except essential services such as hospitals, telecom, pharmacy, provision stores. PM Modi called for 'Janata curfew' on 22nd March, 2020 from 7 AM-9 PM, urging people to stay home except those in essential services, enforcing public-led social distancing interventions. In consultation with medical professionals, detailed advisory has been issued for all health establishments to avoid non-urgent hospitalization and minimize elective surgeries.
Prime Minister interacted with all Chief Ministers to strategize ahead considering extension of lockdown for tackling COVID-19. PMO sanctioned INR 15,000 crores COVID-19 Emergency Response and Health System Preparedness Package; INR 7,774 crores for immediate response and rest medium term support (1-4 years). WHO is supporting Government in assessment of ‘Dedicated COVID-19 hospitals. As of now, Government of India has set up more than 550 dedicated facilities with >1 lakh isolation beds and >11,000 ICU beds have been set up. For capacity building of the front line health workers, AIIMS, New Delhi has been commissioned by Ministry of Health & Family Welfare (MoHFW) for Webinars on COVID-19 together with WHO and partners. Ministry of Home Affairs (MHA) has directed all States/UTs to provide necessary Police Security to Doctors and Medical Staff undertaking surveillance, detection, quarantine and all activities against COVID-19.
As of 17th April, 2020 according to the Ministry of Health & Family Welfare (MoHFW), Govt. of India a total of 11616 COVID-19 cases have been reported in 31 states/union territories. These include 1766 who have been cured, discharged or migrated and 453 deaths. As of 6th May 2021, there have been 21,077,410 confirmed cases of COVID-19, including 411,588 deaths, reported to the Ministry of Health & Family Welfare (MoHFW), Govt. of India.
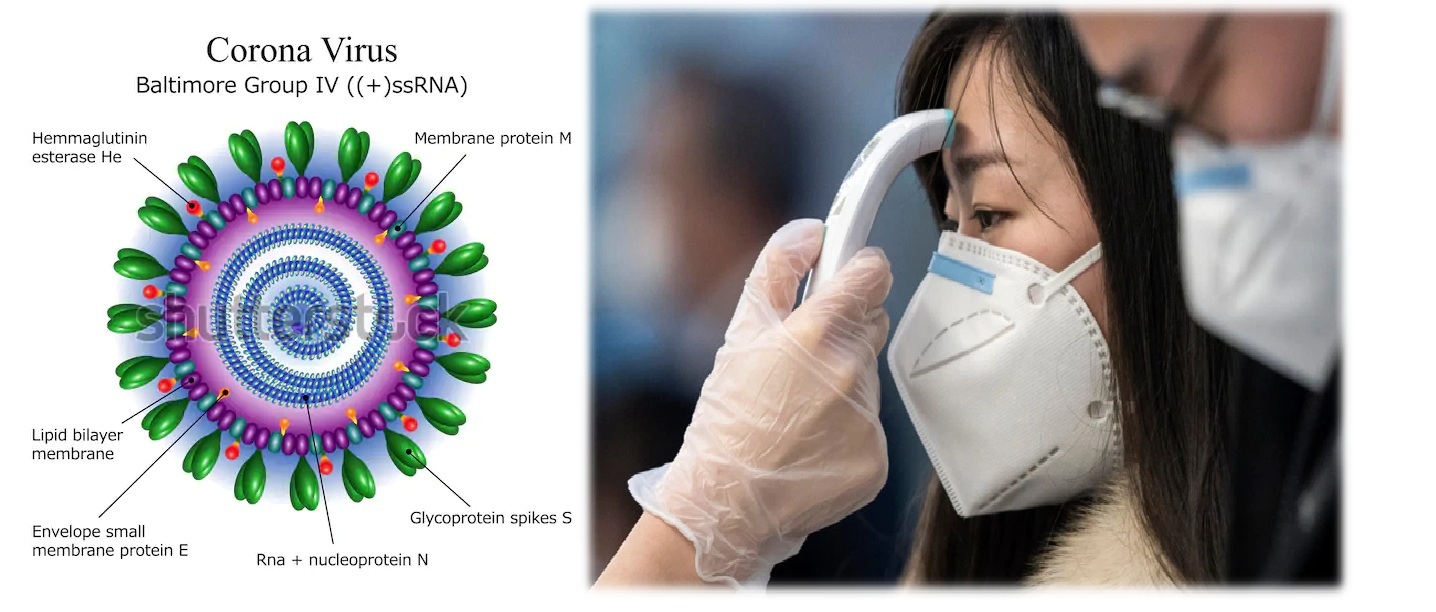 Travelers displaying symptoms of the infection are being separated by the Ministry of Health & Family Welfare’s Integrated Disease Surveillance Programme (IDSP) and directed to isolation facilities. The IDSP has been testing their samples. The Ministry of Health announced on 06 February, 2020 that all 645 evacuees from Wuhan tested negative. Two quarantine canters have been set-up to isolate any passengers showing symptoms of the infection. One centre is located at Manesar, Haryana, and is managed by Armed Forces Medical Services, while the second is located at Chawla Camp in Delhi and is managed by Indo-Tibetan Border Police (ITBP).
Travelers displaying symptoms of the infection are being separated by the Ministry of Health & Family Welfare’s Integrated Disease Surveillance Programme (IDSP) and directed to isolation facilities. The IDSP has been testing their samples. The Ministry of Health announced on 06 February, 2020 that all 645 evacuees from Wuhan tested negative. Two quarantine canters have been set-up to isolate any passengers showing symptoms of the infection. One centre is located at Manesar, Haryana, and is managed by Armed Forces Medical Services, while the second is located at Chawla Camp in Delhi and is managed by Indo-Tibetan Border Police (ITBP).
The National Institute of Virology, Pune, and ten other laboratories under the Indian Council of Medical Research’s (ICMR) Viral Research and Diagnostics Laboratories network are equipped to test samples. The ICMR has tested 510 samples as of 06 February, 2020 of which three tested positive. The Indian government issued a travel advisory on 17 January 2020 to the general public to refrain from travelling to China and avoid contact with anyone with travel history to China since 15 January 2020. The government also temporarily suspended e-Visa facility for Chinese passport holders and noted that already issued e-Visas are temporarily invalid. Online application for physical visa from China has also been suspended. Any persons trying to visit India under compelling circumstances have been advised to contact either the Indian embassy in Beijing or the Indian consulate in Shanghai or Guangzhou.
Mode of Transmission of Coronavirus Infection
Mode of transmission is unknown about 2019-nCoV. Current knowledge is largely based on what is known about similar coronaviruses. Coronaviruses are a large family of viruses that are common in many different species of animals, including camels, cattle, cats, and bats. Rarely, animal coronaviruses can infect people and then spread between people such as with MERS, SARS, and now with 2019-nCoV.
The disease spreads primarily from person to person through small droplets from the nose or mouth, which are expelled when a person with COVID-19 coughs, sneezes, or speaks. These droplets are relatively heavy, do not travel far and quickly sink to the ground. People can catch COVID-19 if they breathe in these droplets from a person infected with the virus, similar to how influenza and other respiratory pathogens spread.
These droplets can land in the mouths or noses of people who are nearby or possibly be inhaled into the lungs; this is why it is important to stay at least 1 meter away from others. Droplet transmission occurs when a person is in close contact (within 1.0 meter) with someone who has respiratory symptoms like coughing or sneezing and is therefore at risk of having his/her mucosae (mouth and nose) or conjunctiva (eyes) exposed to potentially infective respiratory droplets. Transmission may also occur through fomites in the immediate environment around the infected person.
Therefore, transmission of the COVID-19 virus can occur by direct contact with infected people and indirect contact with surfaces in the immediate environment or with objects used on the infected person like prescription, stethoscope, thermometer, tables, doorknobs and handrails etc. It’s commonly happen when a person can get 2019-nCoV by touching a surface or object that has the virus on it and then touching their own mouth, nose, or possibly their eyes; This is why it is important to wash your hands regularly with soap and water or clean with alcohol-based hand rub.
Typically, with most respiratory viruses, people are thought to be most contagious when they are most symptomatic (the sickest). It’s important to note that how easily a virus spreads person-to-person can vary. Some viruses are highly contagious, while other viruses are less so. There is much more to learn about the transmissibility, severity, and other features associated with 2019-nCoV and investigations are ongoing.
Sign & Symptoms of Coronavirus
Coronaviruses typically cause common cold symptoms, but two previously identified beta-coronaviruses, SARS-CoV and MERS-CoV, can result in severe pneumonia and death. Now, a third human coronavirus linked to serious illness — provisionally called 2019-nCoV - is spreading rapidly around the globe after first being described in Wuhan, China. Three reports describe the clinical and epidemiologic features of some of the first cases of this novel infection.
The most common symptoms of COVID-19 are fever, dry cough, and tiredness. Other symptoms that are less common and may affect some patients include aches and pains, nasal congestion, headache, conjunctivitis, sore throat, diarrhoea, loss of taste or smell or a rash on skin or discoloration of fingers or toes. These symptoms are usually mild and begin gradually.
Some people become infected but only have very mild symptoms. Symptoms of 2019-nCoV may appear in as few as 2 days or as long as 14 after exposure. This is based on what has been seen previously as the incubation period of MERS viruses. For confirmed 2019-nCoV infections, reported illnesses have ranged from people with mild symptoms to people being severely ill and dying.
The most common symptoms of COVID-19 are include:
- Fever
- Chills or dizziness
- Cough
- Tiredness
- Shortness of breath or difficulty breathing
- Nasal congestion,
- Conjunctivitis (also known as red eyes)
- Fatigue
- Muscle or body aches
- Headache
- New loss of taste or smell
- Sore throat
- Congestion or runny nose
- Nausea or vomiting
- Diarrhea
Other symptoms that are less common and may affect some patients include:
- Irritability,
- Confusion,
- Reduced consciousness (sometimes associated with seizures),
- Anxiety,
- Depression,
- Sleep disorders
Symptoms of severe COVID‐19 disease include:
- Shortness of breath,
- Loss of appetite,
- Confusion,
- Persistent pain or pressure in the chest,
- High temperature (above 38 °C),
- More severe and rare neurological complications such as strokes, brain inflammation, delirium and nerve damage.
People of all ages who experience fever and/or cough associated with difficulty breathing or shortness of breath, chest pain or pressure, or loss of speech or movement should seek medical care immediately. If possible, call to health care provider, hotline or health facility first, so he/she can be directed to the right clinic.
Diagnostic of Coronavirus Infection
CDC has developed a new laboratory test kit for use in testing patient specimens for 2019 novel coronavirus (2019-nCoV). The test kit is called the “Centers for Disease Control and Prevention (CDC) 2019-Novel Coronavirus (2019-nCoV) Real-Time Reverse Transcriptase (RT)-PCR Diagnostic Panel.” It is intended for use with the Applied Biosystems 7500 Fast DX Real-Time PCR Instrument with SDS 1.4 software. This test is intended for use with upper and lower respiratory specimens collected from persons who meet CDC criteria for 2019-nCoV testing.
CDC’s test kit is intended for use by laboratories designated by CDC as qualified, and in the United States, certified under the Clinical Laboratory Improvement Amendments (CLIA) to perform high complexity tests. The test kits also will be shipped to qualified international laboratories, such as World Health Organization (WHO) Global Influenza Surveillance Response System (GISRS) laboratories. The test will not be available in U.S. hospitals or other primary care settings. The kits will be distributed through the International Reagent Resource external icon (IRR).
On Monday, February 3, 2020, CDC submitted an Emergency Use Authorization (EUA) package to the U.S. Food and Drug Administration (FDA) in order to expedite FDA permitted use of the CDC 2019-nCoV Real-Time RT-PCR Diagnostic Panel in the United States. The EUA process enables FDA to consider and authorize the use of unapproved, but potentially life-saving medical or diagnostic products during a public health emergency. The U.S. Secretary of Health and Human Services declared the 2019-nCoV virus a U.S. public health emergency on Friday, January 31, 2020. FDA issued the EUA on February 4, 2020.
A test to diagnose COVID-19 determines whether the people are actively infected and have the disease. The most common test involves inserting a swab up the nose to take a sample of fluid for testing. Sometimes a throat swab is done instead. A COVID-19 saliva test, recently approved by the FDA, is less risky for the health care worker to collect the sample. The person spits into a plastic tube several times and hands the tube back to the health care worker. The FDA approved one at-home nasal swab kit. After a doctor approves testing as medically appropriate, the person sends the self-collected sample to a lab for testing. With limited kits available at this time, health care workers and first responders get priority. The FDA warns consumers against buying unapproved home tests, because they may be inaccurate and unsafe.
Treatment of Coronavirus Infection
There is no specific antiviral treatment recommended for 2019-nCoV infection. People infected with 2019-nCoV should receive supportive care to help relieve symptoms. For severe cases, treatment should include care to support vital organ functions. At the primary phase of infection, The Drug Controller General of India has granted approval to the Indian Council of Medical Research to use a combination of lopinavir and ritonavir in the event of the coronavirus infection turning into a public health emergency in the country, reported the Economic Times. Lopinavir and ritonavir have already been approved for the treatment of HIV. People who think they may have been exposed to 2019-nCoV should contact your healthcare provider immediately.
The most common symptoms of COVID-19 are fever, chills or dizziness, cough, tiredness, nasal congestion, conjunctivitis (also known as red eyes), fatigue, muscle or body aches, headache, new loss of taste or smell, sore throat, congestion or runny nose, nausea or vomiting and or diarrhea; patient can most likely treat them at home. Most people recover from COVID-19 without the need for hospital care. Call doctor to ask about whether he/she should stay at home or get medical care person.
At-Home Coronavirus Treatment
If symptoms are mild enough that the patient can recover at home, he/she should:
- Complete rest; It can make feel better and may speed recovery.
- Stay home. Don't go to work, school, or public places.
- Drink much more amount fluids; Dehydration can make symptoms worse and cause other health problems.
- Monitoring health status, If symptoms get worse, call the doctor right away. Don't go to their office without calling first. They might tell to stay home, or they may need to take extra steps to protect staff and other patients.
- Ask the doctor about over-the-counter medicines that may help. The most important thing to do is to avoid infecting other people, especially those who are over 65 or who have other health problems.
- Try to stay in one place in your home. Use a separate bedroom and bathroom if possible.
- Wear a mask over your nose and mouth if can.
- Wash regularly, especially your hands.
- Don't share dishes, cups, eating utensils, towels, or bedding with anyone else.
- Clean and disinfect common surfaces like doorknobs, counters, and table tops.
At-Hospital Coronavirus Treatment
Don't need to go to the hospital if person have basic COVID-19 symptoms, like a mild fever or cough on any other common symptoms mention previously. If the patient’s condition is severe, members of the medical staff will check for signs that the illness is causing more serious complication. Then they might
- Check the levels of oxygen in the blood with a pulse-oximeter,
- Examine chest sound (Lungs), a chest X-ray or CT scan.
- If required doctors may give extra oxygen through two small tubes that go just inside nostrils. In very serious cases, doctors will give a ventilator support.
- May also give fluids through IV, to keep from getting dehydrated. Doctors will also closely monitor breathing.
- Doctors may give an antiviral medicine called Remdesivir. Remdesivir is the first drug approved by the FDA for treatment of hospitalized COVID patients over the age of 12 years. Research shows that some patients recover faster after taking it. Remdesivir was created to fight Ebola, but the FDA has issued an emergency use ruling so doctors can use it against COVID-19.
- Doctor might also give other medication to thin the blood and prevent clots. If patient take drugs such as angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), or statins for other health problems; doctor will tell to continue them as usual.
- The FDA has issued an emergency use authorization (EUA) for two drugs called monoclonal antibodies to treat COVID-19. Casirivimab and Imdevimab can be given to high-risk patients who have recently been diagnosed with mild to moderate illness to lower levels of the virus in their bodies and lower the risk of hospitalization.
- Many clinical trials are underway to explore treatments used for other conditions that could fight COVID-19 and to develop new ones. The FDA is also allowing clinical trials and hospital use of blood plasma from people who've recovered from COVID-19 in order to help patients with severe or life-threatening cases (Convalescent plasma).
- Clinical trials are under way for other medications, including tocilizumab, which has been used to treat autoimmune conditions and an inflammatory condition called cytokine release syndrome.
- The FDA has rescinded its emergency authorization for the use of hydroxychloroquine and chloroquine to treat people who are hospitalized with COVID-19 amid serious concerns about their safety and how well they worked against the virus. The medications are approved to treat malaria and autoimmune conditions like rheumatoid arthritis and lupus.
- One study found that dexamethasone, a common steroid medication, can help people who are hospitalized with severe COVID-19 complications. But the findings are preliminary, and the researchers haven’t released the full study.
Scientists are trying to make new medicines and test some existing drugs to see whether they can treat COVID-19. In the meantime, there are a number of things that can relieve symptoms, both at home and at the hospital.
Prevention of Coronavirus Infection
There is currently few vaccines to prevent 2019-nCoV infection. The best way to prevent infection is to avoid being exposed to this virus.
Everyone 16 years of age and older is now eligible to get a COVID-19 vaccination. Get a COVID-19 vaccine as soon as you can. Widespread vaccination is a critical tool to help stop the pandemic.
However, as a reminder, CDC always recommends everyday preventive actions to help prevent the spread of respiratory viruses, including:
- Avoid close contact with people who are sick.
- Avoid touching your eyes, nose, and mouth with unwashed hands.
- Stay home when you are sick.
- Cover your cough or sneeze with a tissue, then throw the tissue in the trash.
- Clean and disinfect frequently touched objects and surfaces using a regular household cleaning spray or wipe.
- Follow CDC’s recommendations for using facemask.
CDC does not recommend that people who are well wear facemask to protect themselves from respiratory viruses, including 2019-nCoV. - Facemask should be used by people who show symptoms of 2019 novel coronavirus, in order to protect others from the risk of getting infected. The use of facemasks is also crucial for health workers and people who are taking care of someone in close settings (at home or in a health care facility).
- Wash your hands often with soap and water for at least 20 seconds, especially after going to the bathroom; before eating; and after blowing your nose, coughing, or sneezing.
- If soap and water are not readily available, use an alcohol-based hand sanitizer with at least 60% alcohol. Always wash hands with soap and water if hands are visibly dirty.
These are everyday habits that can help prevent the spread of several viruses. There is no specific antiviral treatment recommended for 2019-nCoV infection. People infected with 2019-nCoV should receive supportive care to help relieve symptoms. For severe cases, treatment should include care to support vital organ functions.




Love this blog. It’s really very informative…..
Good job! Keep writing…