Osmotic Fragility of Erythrocytes
The red blood cell membrane restricts the solutes and allows only water to pass through them (osmosis). If the cells are placed in hypertonic solution (concentrated sodium chloride solution, concentration more then normal that is > 2.0%), these are sink due to exosmosis. The red cell absorb water when kept in hypotonic solution (solution concentration <0.55% that is below normal saline) due to endosmosis, haemolysis of the cells taken place due to the excessive swelling. In isotonic solution (normal saline – 0.85%) the red cells are stay intact.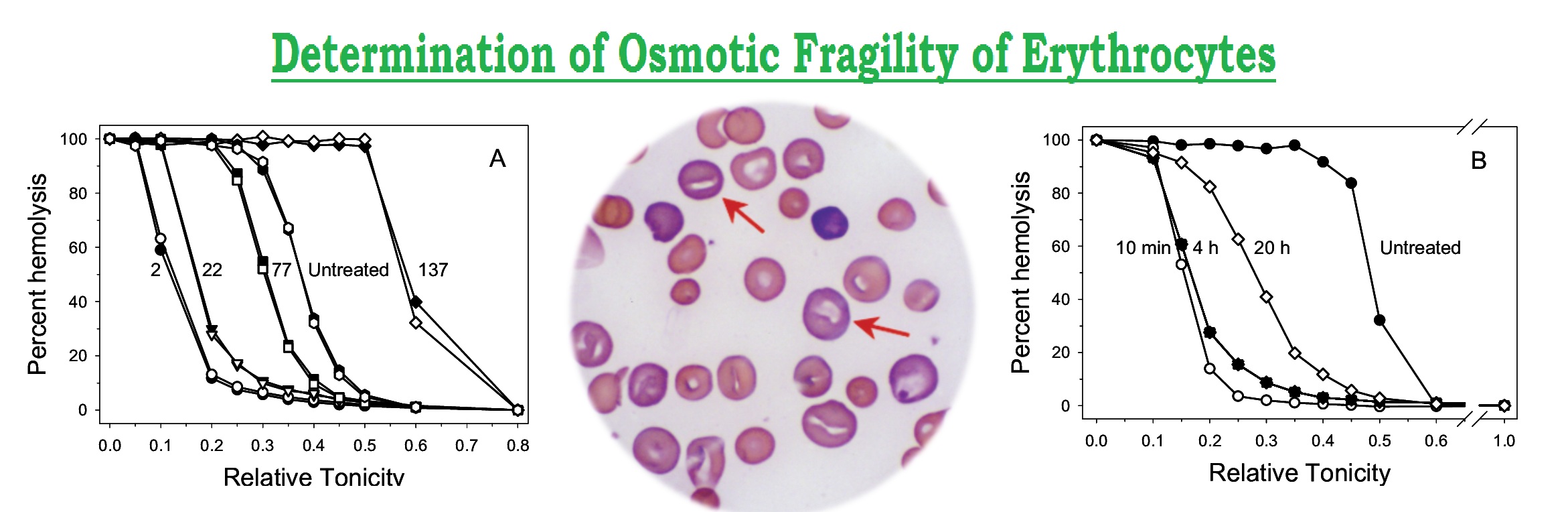
Clinical Significance :
- It is highly increased in hereditary spherocytosis and the red cells are haemolysis at 0.55% sodium chloride solution. Normally, lysis of red cells begins at 0.75% saline and complete lysis occurs at < 45% saline, after splenectomy, red cells become more homogenous and fragility curve returns to normal.
- Decreased osmotic fragility is seen when volume to surface area ratio is decreased with low MCV and MCH in iron deficiency anaemia and thalassaemia. Thalassaemic target cells are haemolyze in below 0.3% sodium chloride solution.
Principle :
A 0.85% sodium chloride solution is isotonic with plasma. The red cells are suspended in decreasing concentration of sodium chloride, ranging from 0.9% to 0.0% (water). Spherocytosis are osmotically fragile and haemolyze more readily then normal cells in hypotonic solution. However, target cells are osmotically resistance. Degree of haemolysis is measured in each tube by using a colorimeter at 540 nm (green filter)
Specimen :
- Heparinized blood or
- Defibrinated blood – For this type specimen, about 10 ml of blood is collected in a conical flash containing 10-15 glass beads. After rotating the blood gently for 5 minutes it is centrifuged to get red cells.
Equipments :
- Test tube with test tube rack.
- 10 ml or 5 ml pipette.
- Graph paper.
- Timer.
- Colorimeter.
- Centrifuge machine.
Reagent :
-
- A) 10 gm/dl Stock Buffer Sodium Chloride :
- Sodium chloride : 90.0 gm
- Na2HPO4 : 13.65 gm
- NaH2PO4, 2 H2O : 2.43 gm
- Distilled water : 1 liter
Due to the presence of phosphate ions the solution is osmotically equivalent to 10 gm/dl.
- B) Preparation of 1.0 gm/dl Buffer :
Preparation of 1.0 gm/dl buffer solution by mixing 10 ml of solution-A and 90 ml of distilled water. - C) Prepared Working Solution :
Now prepared working solution in centrifuge tubes (17 x 120 ml) as follows :
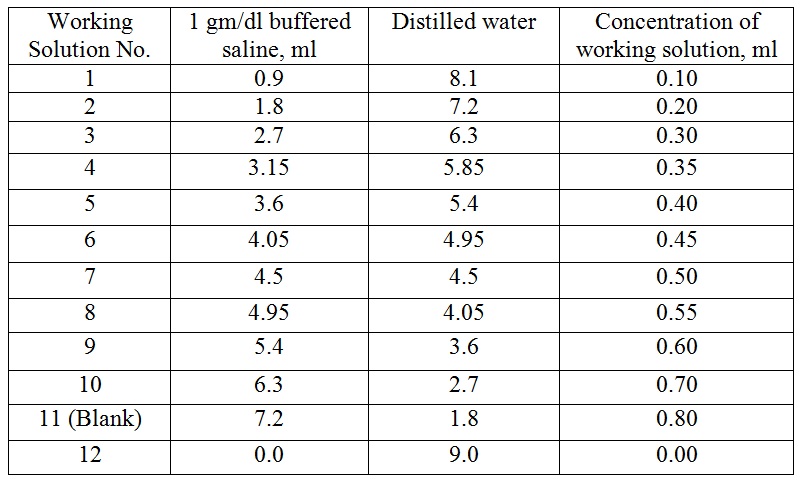 Here, tube no.-11 (blank) gives 0.0% haemolysis and tube no.-12 gives 100% haemolysis.
Here, tube no.-11 (blank) gives 0.0% haemolysis and tube no.-12 gives 100% haemolysis. - D)Preparation of 50% cell suspension in 1 gm/dl of Buffer solution:
- Taken about 5 ml of blood in a graduated centrifuge tube.
- Centrifuge at 1500 r.p.m. for 10 minutes.
- Discard the supernatant and added 2-3 ml of 1 gm/dl NaCl buffered solution. Mixed well and centrifuge at 1500 r.p.m. at 5 minutes.
- Discard the supernatant and repeat the Step-(iii) at least 3 times, discard the supernatant.
- Measured the volume of packed cells and added equal volume of 1 gm/dl buffered saline and mixed well.
- A) 10 gm/dl Stock Buffer Sodium Chloride :
Procedure :
- Added 1 ml of 50% cell suspension to each of the 12 working solution of buffered saline (prepared at step-C ).
- Mixed well and keep all the tubes at room temperature (25C 5C) for 30 minutes.
- Mixed again and centrifuge at 1500 r.p.m. for 10 minutes.
- Read absorbance of the supernatant to each tube at 540 nm (green filter) in the colorimeter by setting blank (tube no.-11) to 100%T.
- Calculation :
 Absorbance with 100% haemolysis is obtained in tube no.-12.
Absorbance with 100% haemolysis is obtained in tube no.-12. - Drawn the fragility curve by plotting percentages of haemolysis on ‘Y’ axis and concentration of buffered solution on ‘X’ axis.
- Determine Median Corpuscular Fragility (MCF) which causes 50% lysis.


Hi there Remarkable! Its really awesome piece of writing, I have got much clear idea regarding from this paragraph. many thanks..
Pretty component of content. I simply stumbled upon your blog and in accession capital to say that I actually loved your blog posts.
Thank you for sharing with us, I think this website genuinely stands out.
So much wonderful information on here. I feel very fortunate to have come across the web page and look forward to many more fabulous times reading here. Thank you once more for all the details.