Rhesus Blood Group System & It’s Variant
In 1940 Landsteiner and Wiener reported that when the red cells of Macacus Rhesus monkeys were introduce into rabbits and guinea pigs, antibodies were evoked. These
caused clumping not only of monkey’s red blood cells but also of the red blood cells of about 85% of human beings. The antigenic factor present on the surface of the human red blood cells called ‘Rh’ antigen.
‘Rh’ Blood group system is the second most important blood group system in human. ‘Rh’ Positive blood cells are antigenic for ‘Rh’ Negative individuals. Introduction of ‘Rh’ Positive cells (antigen) into an ‘Rh’ negative individual will stimulate the production of antibodies (anti-D) which will agglutinate and haemolysed ‘Rh’ Positive cells in 60% -70% cases.
‘Rh’ antibodies are not occurring naturally but develop only after exposure of an individual to antigen which the individuals lacks and the resultant antibody (IgG in nature), is called an immune antibody. These
antibodies act best at 37°C in protein (albumin) medium.
‘Rh’ Antigen :
- Currently adopted nomenclature is Fisher-Race because it is easy to follow.
- The ‘Rh’ blood group system is controlled by five co-dominant, closely linked allelic genes. These genes appear in three pairs – Cc, Dd, Ee.
- Persons whose red cells possess D antigen are called ‘Rh’ Positive (irrespective of the presence or absence of other ‘Rh’ antigen). ‘Rh’ Negative person lacking the D antigen.
- The other four antigen C, E, c, e are present in every individual in some combination. These antigen do not frequently produce strong reacting immune antibodies.
- Rh allelic :
According to the Fisher-Race, three pairs of closely linked allelic genes gives rise to eight possible antigenic combinations - cDe, CDe, cDE, CDE, cde, Cde, cdE, CdE- CDE/CDE (Homozygous)
- CDE/cde (Heterozygous)
- Inheritance of ‘Rh’
group is independent of the ‘ABO’ group. In India approximately 95% population
are ‘Rh’ Positive and 5% are ‘Rh’ Negative. Of ‘Rh’ Negative the frequency of
‘cde’ is higher , while the other pairs (Cde, cdE, cDe) are rare. - Anti-d does not exist
and the presence of ‘d’ is indicated by the absence of ‘D’.
‘Rh’ Antibody :
- Anti-Rh is an IgG immunoglobulin of lower molecular weight (1,70,000).
- These are capable of crossing the placenta and can causes Haemolytic Disease of Newborn (HDN) in ‘Rh’ Negative mother , bearing an ‘Rh’ positive foetus.
- Optimum visible immunologic reaction of these antibody with corresponding antigen take place at 37°C in the presence of protein(albumin).
- About 50-75% of ‘Rh’ negative individual would be capable to produce anti-Rh when immunized with ‘Rh’ Positive cell (antigen), these are called Responders.

Determination of ‘Rh’ Type
Principle :
The procedures based on the principle of agglutination. Normal human red blood cells possessing ‘Rh’ antigen, will clump in the presence of blood typing serum containing corresponding antibody anti-Rh.
Method :
Three methods are used for determination of ‘Rh’ typing. These are as follows :
- Slide method
- Tube method
- Gel method
In the developing country
Slide method is commonly performed. But Special circumstances Tube method as well as Gel method is performed. In the Laboratory We performed Slide method.
Requirements :
- Clean and dry grass free glass slide.
- Pasteur pipette.
- Applicator sticks.
- Centrifuge machine.
- Timer & Marker pen
- Microscope
Reagents :
- Blood typing serum Anti-D
- (Human polygonal or human monoclonal reagents)
- Normal Saline (0.85% NaCl solution)
Specimen :
No special preparation of the patient is required prior to the specimen collection. Blood should be collected by approved technique. The test should be performed immediately. Otherwise it is necessary to store the specimen at 2° - 8°C.
Procedure :
- All the reagents and sample to be brought to room temperature before performing the test.
- On a pre-warmed glass slide (40°C to 50°C surface temperature) placed one drop of Anti-D serum.
- Added one drop of well mixed 10% red blood cells suspension or whole blood (In case of anaemia patient added one drop of sedimented red blood cells) by using a Pasteur pipette.
- Mixed the cell suspension and anti-D by using an applicator stick.
- Tilt the slide back and forth for 2 minutes and observe for agglutination by macroscopically (microscopically if required).
Observation & Interpretation :
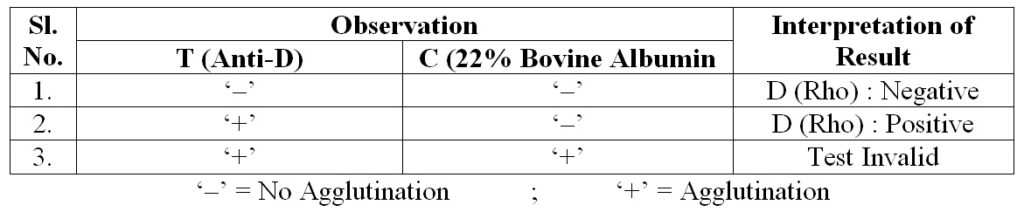
- Agglutination : ‘Rh’ Positive
- No agglutination : ‘Rh’ Negative
Special Note :
- Don’t interpret peripheral drying or fibrin strands as agglutination.
- If No agglutination is observed proceed to Du determination.
- Bovine albumin or ‘Rh’ Negative Controls are not required with human monoclonal anti-D reagents.
Determination of ‘Rh” Typing by Tube method
Procedure :
- Prepared a 5% suspension of red blood cells in a normal saline (0.85% Sodium Chloride Solution).
- Taken a clean and dry test tube and added one drop of Anti-D (‘Rh’ antibody) into the test tube.
- Added one drop of well mixed 5% red blood cell suspension by using a Pasteur pipette.
- Mixed well and centrifuge at 1500 RPM for 1 minute.
- Resuspend the cells by gentle agitation and examine macroscopically and also microscopically for agglutination.
Observation & Interpretation :
- Agglutination : ‘Rh’ Positive
- No agglutination : ‘Rh’ Negative
‘Rh’ VARIANT : Du
Some ‘Rh’ Positive cells fail to show agglutination by the usual tube and slide method and by mistake these can be categorised as ‘Rh’ Negative. Since ‘D’ antigen is expressed weakly. These cells fail to agglutination with anti-D. Additional reaction with Anti Human Globulin (AHG) is necessary to demonstrate agglutination. These red blood cells are known as ‘D’ Variant or Du.
Du Positive cells are considered to be ‘Rh’ Positive; If Du blood is given to a patient, who is truly Negative (dd), its antigenicity will stimulate the production of Anti-D antibody. Hence before blood transfusion of ‘’Rh’ Negative blood, Du test is essential by using Anti Human Globulin. This type of blood from a donor should not be used for transfusion purpose. Recipients, who persist such result must receive only ‘Rh’ Negative blood.
Determination of Du
Principle :
The procedure is based on the principle of agglutination. The cells with Du antigen are sensitized with anti-D by incubating at 37°C. This results in the adsorption of anti-D on the surface of cells without producing haemagglutination (Sensitization). The presence of reacted antibodies on the surface of the Du cells is recognized by using Anti Human Globulin which reacts with the coated antibodies and bring about haemagglutination.
Method :
Two methods are used for determination of ‘Rh’ Variant (Du). These are Tube method and Gel method. In the developing country. Tube method is commonly performed. But Special circumstances Gel method is performed. In the Laboratory We performed Tube method.
Requirements :
- Clean and dry grass free glass test tube.
- Pasteur pipette.
- Applicator sticks.
- Centrifuge machine.
- Timer & Marker pen
- Microscope
- Incubator (fixed temperature 37°C) or Water bath.
Reagent :
- Anti-D Serum
- Anti Human Globulin (AHG)
- 22% Bovine Albumin (Control)
- Normal Saline (0.85% NaCl Solution)
Specimen :
No special preparation of patient is required prior to the specimen collection. EDTA anticoagulated or Clotted blood is required.
Procedure :
- All the reagents and sample to be brought to the room temperature before performing the test.
- Prepared 5% suspension of red blood cells in isotonic solution.
- Labelled one tube as ‘T’ and added in it one drop of anti-D.
- Labelled second tube as ‘C’ and added one drop of 22% Bovine albumin reagent.
- By using a Pasteur pipette, added one drop of cell suspension to each of the two tubes and mixed well.
- Incubated the Test and Control tube for at least 15 minutes at 37°C temperature or placed the tubes in the water-bath for 30 minutes at 37°C (the time depend on the manufacturer’s instruction).
- After incubation, washed the cells in each tube three times with fresh normal saline. And decant the tube completely after the last washing.
- To each tube, added 2 drops of Anti Human Globulin (AHG) [containing anti IgG].
- Mixed the contents of the tube by gently and centrifuged at 1500 RPM for one minute.
- Re-suspended the cells by gentle agitation and examined for agglutination by macroscopically and afterwards microscopically.
Observation
& Interpretation :