Blood Specimen Collection : Processing, Importance, Procedure…..
Blood for diagnostic investigations can be collected either from the peripheral vein or from the capillaries. Venous blood is preferred for hematology, biochemistry, serology & immunology investigations but capillary blood can be nearly as accurate if a free flow of blood is obtained and there is no dilution error due to tissue fluids.
Site Selection
Blood is usually collected from veins, capillaries and arteries. Venous blood is collected from anticubital vein most commonly. Blood is also collected from the vein of arms, dorsum of hands. Long cephalic vein is also used where non-availability of the former veins. Infant’s femoral vein is used for collection of blood. In newborn children umbilical vein and scalp vein are commonly used for puncture.
For collection of capillary blood, the site is selected usually the finger-tips or ear-lobe in adult and the great toe or heel in infants.
Techniques uses for Collection of blood
Mainly two methods are used for collection of blood. :
- Pricking method.
- Venipuncture method.
- Pricking method is used for collection of capillary blood form the finger-tips or earlobe in adult or the big toe or heel in infants.
- Venipuncture method is used for collection of large amount of blood from the vein. The commonly used veins are brachial vein, cephalic vein and anticubital vein in adult and umbilical vein & scalp vein in infants.
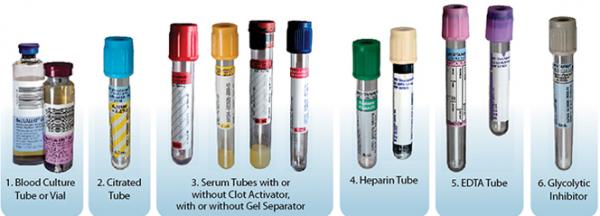
Order of Drawing Blood
Blood collection tubes must be drawn in a specific order to avoid cross-contamination of additives between tubes. The recommended order of draw for plastic vacutainer tubes is:
- First - Blood culture bottle or tube (yellow or yellow-black top)
- Second - Coagulation tube (light blue top).
- Third - non-additive tube (red top)
- Last draw - additive tubes in this order:
NOTE: Tubes with additives must be thoroughly mixed. Clotting or erroneous test results may be obtained when the blood is not thoroughly mixed with the additive.
Areas to Avoid for Collection of Blood
Certain areas are to be avoided when choosing a site for collection of blood:
- Extensive scars from burns and surgery - It's difficult to puncture the scar tissue and obtain a specimen.
- The upper extremity on the side of a previous mastectomy - Test results may be affected because of lymph edema.
- Hematoma - It may cause erroneous test results. If another site is not available, collect the specimen distal to the hematoma.
- Intravenous therapy (IV) / Blood transfusions - Fluid may dilute the specimen, so collect from the opposite arm if possible.
- Cannula/fistula/heparin lock - Hospitals have special policies regarding these devices. In general, blood should not be drawn from an arm with a fistula or cannula without consulting the attending physician.
- Edematous extremities - tissue fluid accumulation alters test results.
Uses of collected blood in Hematology
Uses of capillary blood :
Following tests are performed by using the capillary blood if a free flow of blood is obtained and there is no dilution error due to tissue fluids:
- Hemoglobin estimation.
- Total Leukocyte Count.
- Total Erythrocyte Count.
- Total Platelet Count.
- Differential Leukocyte Count.
- Reticulocyte Count.
- Bleeding Time (BT), Clotting Time (CT)
- ‘ABO’ blood grouping and ‘Rh’ typing.
- Smear for basophilic aggregation.
- Thick & Thin smear for malaria parasite. etc.
Uses of Venous blood :
Following test performed by using venous blood (anticoagulated) in the haematology.
- Hemoglobin estimation.
- Total Leukocyte Count.
- Total Erythrocyte Count.
- Total Platelet Count.
- Differential Leukocyte Count.
- ‘ABO’ blood grouping and ‘Rh’ typing.
- Reticulocyte Count.
- Haematocrit (PCV).
- Erythrocyte Sedimentation Rate (ESR).
- Prothrombin Time (PT).
- Determination of Red Cell Indices etc.
Requirements :
Requirements for Capillary blood collection :
- Lancet or sterile needle (24 G).
- Doctor’s alcohol (70% ethanol)
- Absorbance cotton.
- Capillary tube.
Requirements for venous blood collection :
- Syringe with needle (sterile & disposable).
- Doctor’s alcohol (70% ethanol)
- Absorbance cotton.
- Tourniquet
- Suitable anticoagulated vial.
Blood Collection Procedure
Capillary Blood Collection:
- The site is selected usually the fingertip or earlobe in adults and great toe or heel in infants.
- The selected site is massaged and cleaned with Doctor’s alcohol.
- The skin is air dried because if it remains wet, blood will run all over and not collected into a drop.
- The skin punctured by the sterile lancet or needle sharply about 3 mm of depth. Light pressure arises around the finger and released immediately.
- The first drop of blood is wiped off by using dry absorbance cotton. When a sufficiently large drop has again accumulated, is used for filling the capillary pipette for examination.
- When all the require amount of blood has been collected, the finger should be clean with a cotton swab and pressure applied until bleeding stops.
Venous Blood Collection :
Venous blood is collected either by the syringe method or by the Vacuum tube method. Commonly syringe method is used for collection of blood in all laboratories but Vacuum tube method is also used for special circumstance.
Use of Syringe method for the collection of venous blood.
- The patient set comfort condition.
- The patient’s prescriptions observe and calculate the amount of blood is required for total examination, describe in prescription.
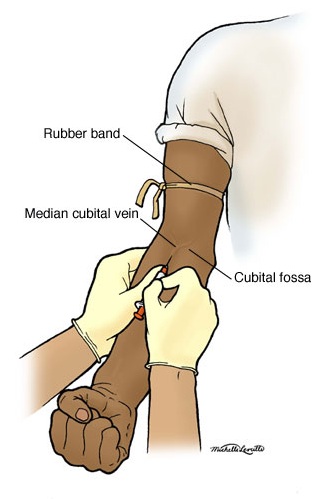
- The upper part of the arm is constricted by applying a tourniquet to hinder venous return with out obstructing the radial pulse.
- The volar region on the vein (foreman area) is clean with 70% ethyl alcohol (Doctor’s alcohol) and the patient to clench the hand to make the vein prominent.
- The vein is fixed by slight traction and the needle with the bevel upwards is inserted at an angle of 15º - 25º to the skin.
- The piston of the syringe is move back-ward; hence blood is enter in the syringe.
- After collection of require amount of blood, the tourniquet is released and the needle is quickly with-drawn. Pressure is applied with cotton over the puncture site.
- Blood is transferred to the appropriate anticoagulated vial and mixed the blood with anticoagulant immediately.
- Told the patient to wait for few minutes, when blood flowing stopped then cover the puncture area by using banded.
The following conditions are done when peripheral blood is freely flowing after skin puncture.:
- PCV, RBC count and haemoglobin concentration of peripheral blood are slightly higher then venous blood.
- The Total Leukocyte Count and Neutrophil count is higher by about 8% and Monocyte may be higher by about 12%.
- Platelet count appear to be higher in venous blood then in peripheral blood an average by about 9% and in some cases by as much as 32% . This may be due to adhesion of platelets to the site of the skin puncture.
Techniques to Prevent Haemolysis :
- Mix all tubes with anticoagulant additives gently (vigorous shaking can cause haemolysis) about 5-10 times.
- Avoid drawing blood from a hematoma; select another draw site.
- If using a needle and syringe, avoid drawing the plunger back too forcefully.
- Make sure the venipuncture site is dry before proceeding of Collection.
- Avoid a probing, traumatic venipuncture.
- Avoid prolonged tourniquet application (no more than 2 minutes; less than 1 minute is optimal).
- Avoid massaging, squeezing or probing a site.
- Avoid excessive fist clenching.
- If blood flow into tube slows, adjust needle position to remain in the center of the lumen.
Blood Sample Handling and Processing:
Pre-centrifugation Handling : The first critical step in the lab testing process, after obtaining the sample, is the preparation of the blood samples. Specimen integrity can be maintained by following some basic handling processes:
- Fill tubes to the stated draw volume to ensure the proper blood-to-additive ratio. Allow the tubes to fill until the vacuum is exhausted and blood flow ceases.
- Vacutainer tubes should be stored at 4-25°C (39-77°F).
- Tubes should not be used beyond the designated expiration date.
- Mix all gel barrier and additive tubes by gentle inversion 5 to10 times immediately after the draw. This assists in the clotting process. This also assures homogenous mixing of the additives with the blood in all types of additive tubes.
- Serum separator tubes should clot for a full 30 minutes in a vertical position prior to centrifugation. Short clotting times can result in fibrin formation, which may interfere with complete gel barrier formation.
Blood Sample Centrifugation : It is recommended that serum be physically separated from contact with cells as soon as possible, with a maximum time limit of 2 hours from the time of collection.
- Complete gel barrier formation (gel barrier tubes) is time, temperature and G-force dependent. The uniformity of the barrier is time dependent; an incomplete barrier could result from shortened centrifugation times.
- In general, for a horizontal, swing-bucket centrifuge, the recommended spin time is 10 minutes. For a fixed-angle centrifuge, the recommended spin time is 15 minutes.
- Gel flow may be impeded if chilled before or after centrifugation.
- Tubes should remain closed at all times during the centrifugation process.
- Place the closed tubes in the centrifuge as a “balanced load” noting the following:
- Opposing tube holders must be identical and contain the same cushion or none at all.
- Opposing tube holders must be empty or loaded with equally weighted samples (tubes of the same size and equal in fill).
- If an odd number of samples is to be spun, fill a tube with water to match the weight of the unpaired sample and place it across from this sample.
Special Note for Centrifuge Safety
- Interference with an activated centrifuge by an impatient employee can result in bodily injury in the form of direct trauma or aerosolization of hazardous droplets.
- Centrifuges must never be operated without a cover in place.
- Uncovered specimen tubes must not be centrifuged.
- Centrifuges must never be slowed down or stopped by grasping part(s) of the device with your hand or by applying another object against the rotating equipment.
- Be sure the centrifuge is appropriately balanced before activating. If an abnormal noise, vibration, or sound is noted while the centrifuge is in operation, immediately stop the unit (turn off the switch) and check for a possible load imbalance.
- Clean the centrifuge daily with a disinfectant and paper towel. Broken tubes or liquid spills must be cleaned immediately.


Pretty nice post. I just stumbled upon your post and wished to say that I’ve really enjoyed browsing your article. After all I’ll be subscribing to your rss feed and I hope you write again soon!
Hello ! I want to say that this article is awesome, nice written and include almost all important infos. I would like to see more posts like this .
Hey there, You’ve done a fantastic job. I definitely study it and personally recommend to my friends.
I’m confident they will be benefited from this website.
Good day, I am so thrilled I found your site, I really found you by error, while I was searching on Bing for something else, Anyways I am here now and would just like to say thanks a lot for a marvelous post and a all round thrilling blog, Please do keep up the great job.